Access to and outcomes of HCT for adult underserved populations have improved over time, but African American and other pediatric patients still experience a significant disparity
Dec 2021
According to research presented at the 63rd American Society of Hematology (ASH) Annual Meeting and Exposition, rates of autologous and allogeneic hematopoietic cell transplants (HCT) have increased more rapidly among African American, Hispanic, and other underserved populations than among non-Hispanic whites from 2009 to 2018. However, a challenge still exists to close the gaps in overall survival for African American and other pediatric patients.
There has been an increase in autologous and allogeneic HCT worldwide and improvement in outcomes after HCT for most hematologic disorders over time. Racial and ethnic disparities in access and outcomes of HCT have been well documented, with substantial effort taken to mitigate these disparities. This research looked at whether there have been improvements in the utilization and outcomes after HCT between whites and underserved populations over time.
The study used data from the CIBMTR® (Center for International Blood and Marrow Transplant Research®) on all patients for any disease who had their first HCT between 2009 and 2018 and consented for research through the CIBMTR. The CIBMTR is a research collaboration between the National Marrow Donor Program®/NMDPSM and Medical College of Wisconsin.
Provider reported race and ethnicity were divided into non-Hispanic white (NWH), African American (AA), Hispanic and other (Asian, Pacific Islander and Native American). Individuals who had missing race/ethnicity information or had a syngeneic transplant (identical twin donor) were excluded. The total sample included 80,080 patients who received an autologous HCT and 60,412 patients who received an allogeneic HCT.
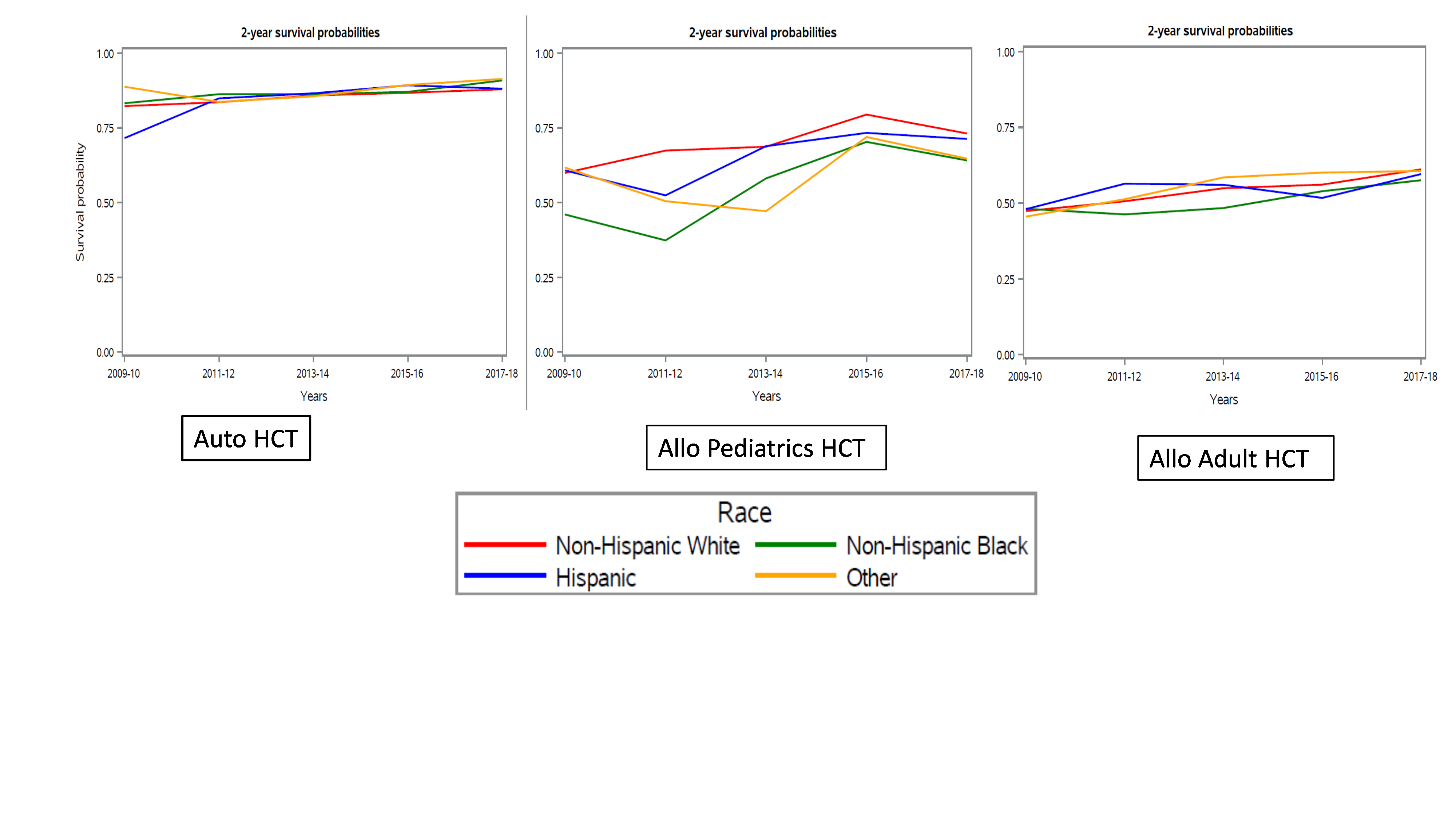
Results showed a higher rate of increase in the volume of HCTs for AA and Hispanic patients compared to NHW patients, and higher rate of increase than the rate of change in U.S. population growth. This suggests progress in closing disparities and gaps to HCT access. Improvement was seen in all groups, and no significant difference in 2-year chance of survival after HCT for adults was observed between any racial or ethnic group and NHW patients. However, researchers observed a worse chance of 2-year survival was observed across all time points for pediatric patients who were AA or other races or ethnicities undergoing allogeneic HCT compared to NHW patients.
This large population-based study highlights the improvement in the volumes and outcomes of HCT in adults over time. Progress is reflected in higher rates of increase in racial/ethnic minority groups and comparable survival across these groups compared to two prior registry studies. In pediatric patients, however, there is a continued gap in outcomes between AA and NHW patients. The researchers express a need for further understanding as to why disparities persist and a continued focus on improving access and outcomes to HCT.
Figure 1. 2-Year Survival Outcomes by Race and Ethnicity
Khera N, et al., ASH oral presentation abstract