Cryopreservation relieves access issues for patients needing HCT during the COVID-19 pandemic
March 2022
According to research presented at the 2022 Tandem Meetings of the American Society of Transplantation and Cellular Therapy (ASTCT) and the CIBMTR® (Center for International Blood and Marrow Transplant Research®), the NMDP Cryopreservation program was effective in ensuring access to hematopoietic stem cell transplant (HCT) by supporting cryopreservation of stem cell and bone marrow products throughout the COVID-19 pandemic.
Full study summary with journal citation (PDF)
The COVID-19 pandemic’s impact on travel, transportation and resources management restricted transplant center access to fresh stem cell and bone marrow products for patients needing transplants. This necessitated using cryopreservation for stem cell and bone marrow products. To ensure access to cryopreservation for transplant programs lacking capacity, resources or expertise, NMDP provides cryopreservation services through NMDP Cryopreservation in partnership with two network cell therapy labs: the Carolinas Cord Blood Bank at Duke University and the Molecular and Cellular Therapeutics Program at the University of Minnesota. Products cryopreserved through Cryopreservation are collected and transported to either lab for cryopreservation and characterization. The products remain in cold storage until requested by the treating transplant program. Additionally, products cryopreserved through the Cryopreservation program can be consented for use by an alternative future patient if unused by the initially intendeded patient, reducing wasted donor product.
Researchers assessed all Cryopreservation products from January 2020 to February 2022. Transport time from collection to cryopreservation is largely within 24 hours (mean 16.3 hours, median 17.2 hours, n=80). To date, over 140 Cryopreservation products have been provided to over 50 transplant programs. Demographics for 55 patients from 28 transplant centers were available through CIBMTR. In the 24 recipients who had engraftment data reported, 92% achieved successful neutrophil engraftment (see Figures below). This preliminary data supports that cryopreservation through a centralized program like Cryopreservation results in successful engraftment.
Cryopreservation supports the transplant community by providing centralized cryopreservation services. As a registry-sponsored service, benefits can include greater flexibility in scheduling product collection, decreasing donor burden and improving donor availability. In addition, for transplant centers and patients, this model increases serviceability in terms of product transport, processing speed and confidence in obtaining a quality stem cell or bone marrow product when limited cryopreservation capacity is available or during times of global uncertainty.
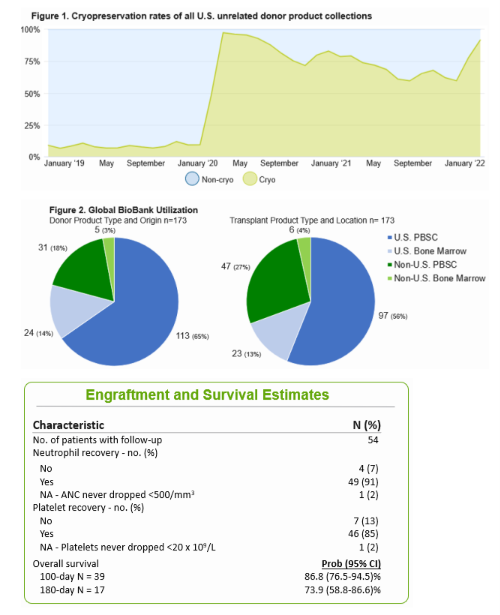
Wren J, et al., Tandem poster abstract