COVID-19 vaccination is effective and recommended 3 months after allogeneic HCT
Apr 2023
This prospective study co-sponsored by the CIBMTR® (Center for International Blood and Marrow Transplant Research®) and the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) aimed to investigate the safety and immunogenicity of COVID-19 vaccination in patients within the first year following allogeneic hematopoietic cell transplantation (alloHCT).
The results support the current guidelines of initiating vaccination 3 months after alloHCT, showing that vaccination is safe and elicits a similar immune response regardless of whether it's administered earlier ( <4 months) or later (4–12 months) post-transplant. However, immune responses were less robust than non-immunocompromised individuals, indicating the need for supplementary preventive and treatment strategies.
Download a PDF of the study highlights and citation.
Background
Patients with blood cancers, especially those undergoing alloHCT, are at high risk of severe COVID-19 complications and death due to SARS-CoV-2 infection. With approximately 12,000 alloHCT procedures performed annually in the United States, the growing population of immunocompromised individuals is particularly vulnerable to severe COVID-19.
These patients were not included in initial vaccine trials, resulting in limited data about vaccine efficacy in this high-risk group. To fill this data gap, the CIBMTR and BMT CTN conducted a comprehensive study on the timing of vaccine administration post-transplant and the associated development of protective immunity.
Study Details
This study involved patients of all ages undergoing alloHCT and scheduled to receive their initial post-transplant SARS-CoV-2 vaccination within one year of the procedure. Recruitment included alloHCT recipients (n=175) from 22 U.S. centers from April to November 2021. Of these recipients, 43% were vaccinated within 4 months of alloHCT, and 57% were vaccinated between 4 and 12 months post-alloHCT.
Demographics were consistent across both groups, with more than 65% who underwent alloHCT due to myeloid diseases, a median age of 58, and predominantly male (54%) and white (85%). The early vaccination group included more recent alloHCT recipients, greater use of peripheral blood stem cells, and fewer instances of chronic graft-versus-host (cGVHD) disease before vaccination.
Results
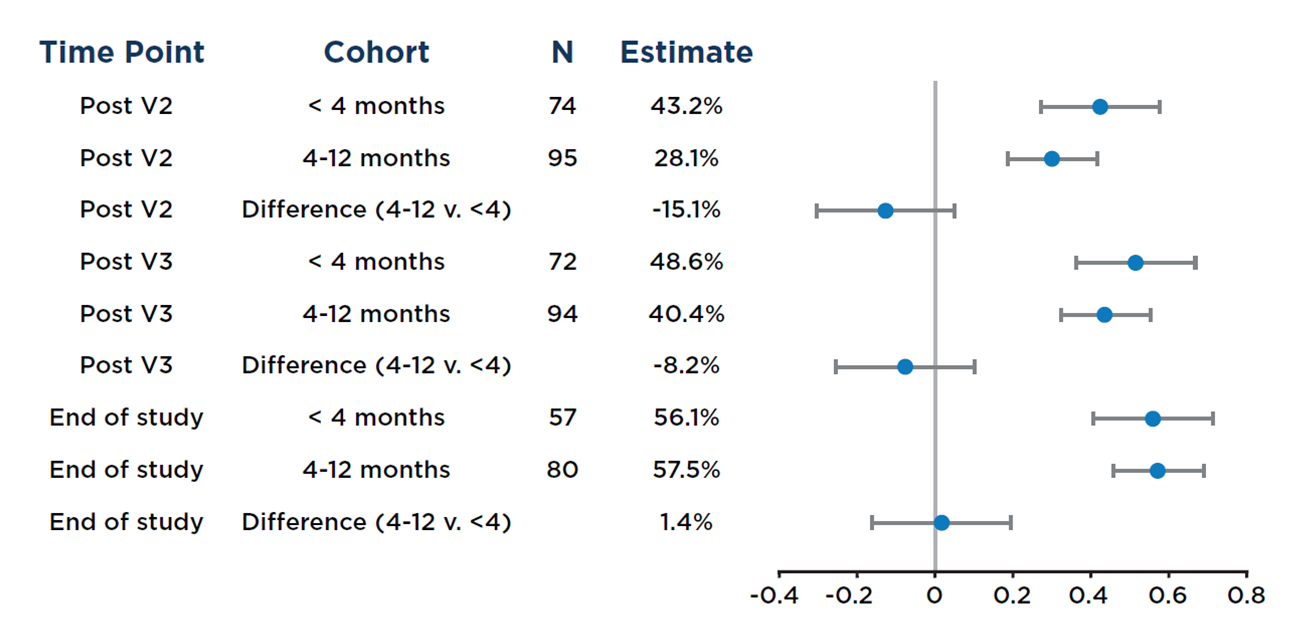
The study found that in alloHCT recipients who received mRNA SARS-CoV-2 vaccines within their first year post-transplant, both humoral (antibody) and cellular responses after two or more vaccinations were similar regardless of whether vaccinations started less than 4 months or between 4 and 12 months post-transplant (see Figure below).
Results also showed that a specific level of anti-spike protein IgG antibodies (titers ≥2403 U/mL) in the blood was associated with an effective immune response, mirroring what's observed in non-immunocompromised vaccine recipients. Most participants achieving this antibody level also exhibited SARS-CoV-2-specific cellular immune responses.
Key Takeaways
The study indicates that COVID-19 vaccination within the first year post-transplant is safe and triggers comparable immune responses irrespective of whether the vaccine is administered earlier or later after HCT. The immune response, however, was lower than in non-immunocompromised individuals, emphasizing the need for additional preventative measures and treatment strategies. The vaccine response was not significantly affected by GVHD treatments or the continued use of immunosuppressive medications. Based on these findings, it's recommended that COVID-19 vaccination be initiated 3 months after alloHCT.
Figure 1. Forest plot of the proportion of individuals at each time point, stratified by vaccine initiation <4 months versus 4–12 months after allogeneic HCT, who had a positive anti-S response; Wald 99% confidence intervals (CI) are shown
Hill JA, et al., Published in eClinical Medicine