HCT provides a significant survival benefit for patients with MDS aged 50 to 75, with no negative impacts on quality of life
December 2021
Outcomes of allogeneic hematopoietic cell transplantation (alloHCT) among selected older patients with advanced myelodysplastic syndrome (MDS) are similar to younger patients and result in no worse quality of life (QOL). Researchers presented these findings at the 62nd and 63rd American Society of Hematology (ASH) Annual Meeting and Exposition.
Download the full study summary with journal citation for:
- Community hematologists/oncologists and referring physicians (PDF)
- Transplant center physicians (PDF)
This multi-center biologic assignment trial conducted by the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) compared the use of HCT over non-HCT therapy in older patients with high-risk MDS. The study was first highlighted at the 62nd ASH Annual Meeting (December 2020) and showcased a significant overall survival (OS) advantage for Int-2 and High IPSS risk de novo patients ages 50 to 75 who received reduced intensity conditioning (RIC) before HCT and had a suitable HLA-matched (8/8) donor.
The benefit of having a matched donor was seen across all subgroups, including those who were of Medicare age. Currently, alloHCT remains the only curative therapy for MDS, but is infrequently offered to older patients and is only available to Medicare patients that enroll in a clinical trial.
Balanced for age, gender, KPS, IPSS risk, MDS disease duration, and response to hypomethylating therapy, 384 subjects (donor n=260, no donor n=124) at 34 centers were enrolled in the study between January 2014 and November 2018. Individuals joined prior to initiation of a formal donor search, and before or after MDS treatment was introduced. All patients were initially assigned to the no donor arm; however, if a suitable donor was identified within 90 days, they were reassigned to the donor arm.
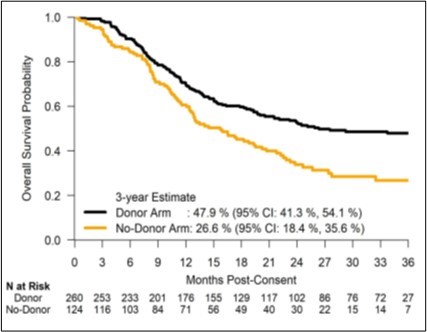
The adjusted OS at 3 years for the donor arm was 47.9% compared with 26.6% in the no donor arm with an absolute difference of 21.3%. The leukemia-free survival (LFS) at 3 years was 35.8% in the donor arm compared with 20.6% in the no donor arm. Survival benefit was seen across all subgroups tested.
Figure 1. Overall survival probability
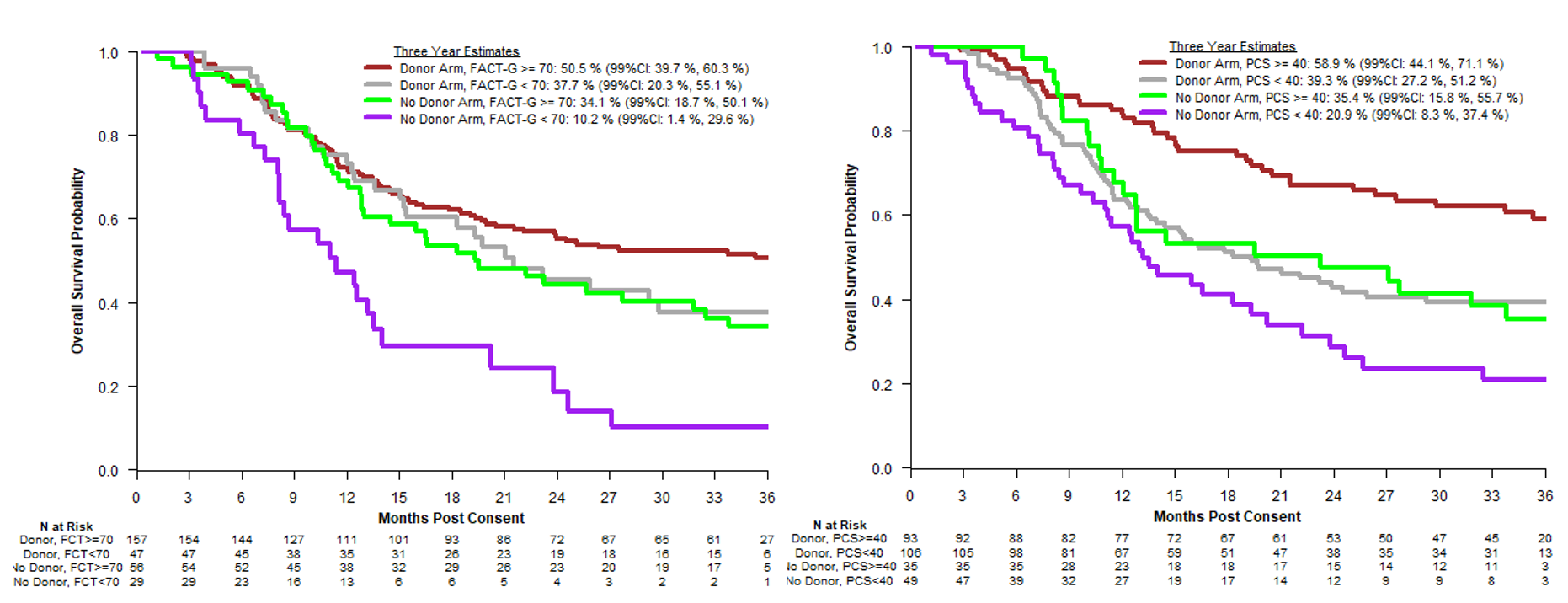
Highlighted at the 63rd Annual ASH Meeting (December 2021), researchers further explored QOL as the secondary outcome in this study. Using patient-reported outcomes offered in English and Spanish, researchers analyzed QOL based on the Functional Assessment of Cancer Therapy General scale (FACT-G) and the Physical Component Score (PCS), assessing measures of perceived physical functionality, bodily pain, and general health. A Mental Component Score (MCS) was also assessed to examine mental health, social function, and vitality. QOL measures were taken at the time of enrollment, as well as at 6, 12, 18, 24, and 36 months post study enrollment.
Results showed similar trajectories for QOL for both the donor and no donor study arms, decreasing or stable from baseline to 6 months with some improvement thereafter. Older patients in the donor arm who underwent HCT were not seen to experience any worse QOL outcomes than those without donors, and in fact, there were some differences at 18 month follow up that favored the donor arm for better QOL, specifically for bodily pain and general health. Baseline QOL was found to be a consistent predictor of survival outcomes. Researchers, therefore, concluded that HCT should be offered to patients ages 50-75 with a suitable donor.
Figure 2. Quality of Life Associations with Overall Survival
62nd ASH Annual Meeting: Nakamura R, et al., ASH oral presentation abstract
63rd ASH Annual Meeting:
Martens M, et al., ASH oral presentation abstract